The Size Of A Molecule
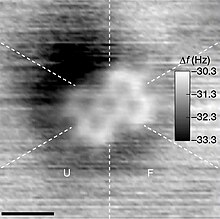


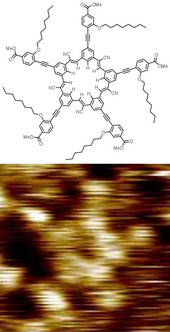
AFM image of i,v,nine-trioxo-xiii-azatriangulene and its chemic structure.[3]
A molecule is a grouping of two or more atoms held together by bonny forces known every bit chemical bonds; depending on context, the term may or may not include ions which satisfy this benchmark.[4] [v] [6] [vii] [8] In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
A molecule may be homonuclear, that is, it consists of atoms of i element, due east.g. 2 atoms in the oxygen molecule (O2); or information technology may be heteronuclear, a chemic compound equanimous of more than ane chemical element, e.g. water (two hydrogen atoms and ane oxygen atom; H2O). In the kinetic theory of gases, the term molecule is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains 2 or more than atoms, since the noble gases are individual atoms.[9] Atoms and complexes continued by not-covalent interactions, such as hydrogen bonds or ionic bonds, are typically non considered single molecules.[10]
Concepts like to molecules accept been discussed since aboriginal times, merely modern investigation into the nature of molecules and their bonds began in the 17th century. Refined over fourth dimension by scientists such as Robert Boyle, Amedeo Avogadro, Jean Perrin, and Linus Pauling, the study of molecules is today known every bit molecular physics or molecular chemical science.
Etymology
According to Merriam-Webster and the Online Etymology Lexicon, the discussion "molecule" derives from the Latin "moles" or small unit of mass. The word is derived from French molécule (1678), from New Latin molecula, atomic of Latin moles "mass, barrier". The word, which until the late 18th century was used just in Latin form, became pop later being used in works of philosophy by Descartes.[eleven] [12]
History
The definition of the molecule has evolved as knowledge of the structure of molecules has increased. Earlier definitions were less precise, defining molecules every bit the smallest particles of pure chemic substances that still retain their composition and chemical properties.[thirteen] This definition oftentimes breaks down since many substances in ordinary feel, such as rocks, salts, and metals, are composed of large crystalline networks of chemically bonded atoms or ions, simply are not fabricated of discrete molecules.
The modernistic concept of molecules tin be traced back towards pre-scientific and Greek philosophers such as Leucippus and Democritus who argued that all the universe is equanimous of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (burn down (![]() ), earth (
), earth (![]() ), air (
), air (![]() ), and water (
), and water (![]() )) and "forces" of attraction and repulsion allowing the elements to interact.
)) and "forces" of attraction and repulsion allowing the elements to interact.
A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, forth with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe.
In a more than concrete manner, still, the concept of aggregates or units of bonded atoms, i.e. "molecules", traces its origins to Robert Boyle'due south 1661 hypothesis, in his famous treatise The Sceptical Chymist, that matter is composed of clusters of particles and that chemical change results from the rearrangement of the clusters. Boyle argued that affair's basic elements consisted of various sorts and sizes of particles, called "corpuscles", which were capable of arranging themselves into groups. In 1789, William Higgins published views on what he called combinations of "ultimate" particles, which foreshadowed the concept of valency bonds. If, for example, according to Higgins, the force betwixt the ultimate particle of oxygen and the ultimate particle of nitrogen were 6, then the strength of the strength would exist divided accordingly, and similarly for the other combinations of ultimate particles.
Amedeo Avogadro created the word "molecule".[14] His 1811 paper "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to Partington's A Short History of Chemistry, that:[15]
The smallest particles of gases are not necessarily uncomplicated atoms, but are made upward of a certain number of these atoms united by attraction to course a single molecule.
In coordination with these concepts, in 1833 the French chemist Marc Antoine Auguste Gaudin presented a clear account of Avogadro's hypothesis,[xvi] regarding diminutive weights, past making use of "volume diagrams", which conspicuously evidence both semi-correct molecular geometries, such every bit a linear water molecule, and correct molecular formulas, such equally H2O:
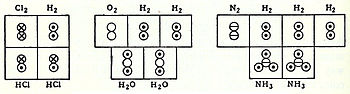
Marc Antoine Auguste Gaudin's volume diagrams of molecules in the gas phase (1833)
In 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method at the Oregon Agricultural College, which was the mainstream description of bonds betwixt atoms at the time. Pauling, notwithstanding, wasn't satisfied with this method and looked to the newly emerging field of quantum physics for a new method. In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the beingness of molecules. He did this by calculating Avogadro's number using iii dissimilar methods, all involving liquid phase systems. First, he used a gamboge soap-similar emulsion, 2nd past doing experimental work on Brownian movement, and third past confirming Einstein's theory of particle rotation in the liquid phase.[17]
In 1927, the physicists Fritz London and Walter Heitler applied the new quantum mechanics to the deal with the saturable, nondynamic forces of attraction and repulsion, i.e., exchange forces, of the hydrogen molecule. Their valence bond treatment of this problem, in their joint paper,[18] was a landmark in that it brought chemistry under quantum mechanics. Their piece of work was an influence on Pauling, who had but received his doctorate and visited Heitler and London in Zürich on a Guggenheim Fellowship.
Subsequently, in 1931, edifice on the work of Heitler and London and on theories institute in Lewis' famous article, Pauling published his footing-breaking article "The Nature of the Chemical Bail"[19] in which he used quantum mechanics to calculate properties and structures of molecules, such equally angles between bonds and rotation about bonds. On these concepts, Pauling adult hybridization theory to account for bonds in molecules such as CHiv, in which four sp³ hybridised orbitals are overlapped by hydrogen's 1s orbital, yielding four sigma (σ) bonds. The 4 bonds are of the same length and strength, which yields a molecular construction as shown below:
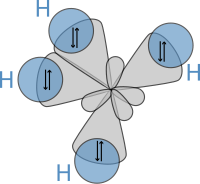
A schematic presentation of hybrid orbitals overlapping hydrogens' s orbitals
Molecular science
The scientific discipline of molecules is called molecular chemistry or molecular physics, depending on whether the focus is on chemistry or physics. Molecular chemical science deals with the laws governing the interaction between molecules that results in the germination and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. In practice, however, this distinction is vague. In molecular sciences, a molecule consists of a stable organisation (bound land) composed of two or more atoms. Polyatomic ions may sometimes be usefully thought of as electrically charged molecules. The term unstable molecule is used for very reactive species, i.e., short-lived assemblies (resonances) of electrons and nuclei, such as radicals, molecular ions, Rydberg molecules, transition states, van der Waals complexes, or systems of colliding atoms as in Bose–Einstein condensate.
Prevalence
Molecules as components of matter are common. They likewise make up most of the oceans and temper. About organic substances are molecules. The substances of life are molecules, e.g. proteins, the amino acids of which they are composed, the nucleic acids (Dna and RNA), sugars, carbohydrates, fats, and vitamins. The nutrient minerals are by and large ionic compounds, thus they are not molecules, e.thousand. iron sulfate.
Withal, the majority of familiar solid substances on Globe are made partly or completely of crystals or ionic compounds, which are not made of molecules. These include all of the minerals that make up the substance of the Earth, sand, dirt, pebbles, rocks, boulders, bedrock, the molten interior, and the core of the Earth. All of these contain many chemic bonds, merely are not made of identifiable molecules.
No typical molecule can exist divers for salts nor for covalent crystals, although these are often composed of repeating unit cells that extend either in a plane, eastward.g. graphene; or three-dimensionally e.g. diamond, quartz, sodium chloride. The theme of repeated unit of measurement-cellular-structure also holds for most metals which are condensed phases with metallic bonding. Thus solid metals are not fabricated of molecules. In spectacles, which are solids that exist in a vitreous matted country, the atoms are held together by chemical bonds with no presence of any definable molecule, nor whatsoever of the regularity of repeating unit of measurement-cellular-construction that characterizes salts, covalent crystals, and metals.
Bonding
Molecules are mostly held together past covalent bonding. Several non-metallic elements be just as molecules in the environment either in compounds or as homonuclear molecules, not equally free atoms: for example, hydrogen.
While some people say a metallic crystal tin can be considered a single giant molecule held together by metallic bonding,[xx] others indicate out that metals behave very differently than molecules.[21]
Covalent

A covalent bail forming H2 (right) where two hydrogen atoms share the two electrons
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are termed shared pairs or bonding pairs, and the stable rest of bonny and repulsive forces between atoms, when they share electrons, is termed covalent bonding.[22]
Ionic
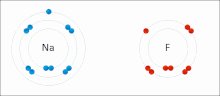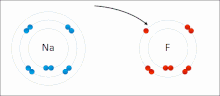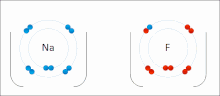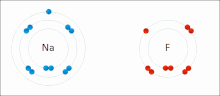
Ionic bonding is a type of chemical bond that involves the electrostatic allure betwixt oppositely charged ions, and is the primary interaction occurring in ionic compounds. The ions are atoms that have lost ane or more electrons (termed cations) and atoms that accept gained ane or more electrons (termed anions).[23] This transfer of electrons is termed electrovalence in dissimilarity to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal cantlet, but these ions can be of a more complicated nature, e.m. molecular ions similar NH4 + or Then4 2−. At normal temperatures and pressures, ionic bonding more often than not creates solids (or occasionally liquids) without divide identifiable molecules, simply the vaporization/sublimation of such materials does produce separate molecules where electrons are notwithstanding transferred fully plenty for the bonds to be considered ionic rather than covalent.
Molecular size
Nigh molecules are far likewise minor to be seen with the naked eye, although molecules of many polymers can reach macroscopic sizes, including biopolymers such every bit DNA. Molecules commonly used as edifice blocks for organic synthesis take a dimension of a few angstroms (Å) to several dozen Å, or effectually one billionth of a meter. Single molecules cannot commonly be observed by lite (as noted above), but minor molecules and even the outlines of individual atoms may be traced in some circumstances by apply of an atomic force microscope. Some of the largest molecules are macromolecules or supermolecules.
The smallest molecule is the diatomic hydrogen (H2), with a bail length of 0.74 Å.[24]
Effective molecular radius is the size a molecule displays in solution.[25] [26] The table of permselectivity for different substances contains examples.
Molecular formulas
Chemic formula types
The chemical formula for a molecule uses i line of chemic chemical element symbols, numbers, and sometimes as well other symbols, such as parentheses, dashes, brackets, and plus (+) and minus (−) signs. These are limited to one typographic line of symbols, which may include subscripts and superscripts.
A compound's empirical formula is a very simple type of chemical formula.[27] It is the simplest integer ratio of the chemic elements that establish information technology.[28] For case, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms, and ethanol (ethyl alcohol) is e'er composed of carbon, hydrogen, and oxygen in a ii:half dozen:1 ratio. All the same, this does not determine the kind of molecule uniquely – dimethyl ether has the same ratios as ethanol, for instance. Molecules with the aforementioned atoms in different arrangements are called isomers. Besides carbohydrates, for example, have the same ratio (carbon:hydrogen:oxygen= 1:2:i) (and thus the same empirical formula) but dissimilar full numbers of atoms in the molecule.
The molecular formula reflects the exact number of atoms that compose the molecule and so characterizes different molecules. Nevertheless dissimilar isomers can have the same atomic composition while being different molecules.
The empirical formula is often the same every bit the molecular formula only non ever. For example, the molecule acetylene has molecular formula C2H2, but the simplest integer ratio of elements is CH.
The molecular mass can be calculated from the chemical formula and is expressed in conventional atomic mass units equal to 1/12 of the mass of a neutral carbon-12 (12C isotope) atom. For network solids, the term formula unit of measurement is used in stoichiometric calculations.
Structural formula
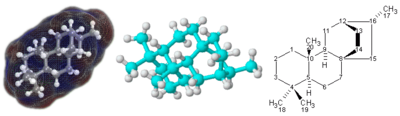
3D (left and center) and 2D (correct) representations of the terpenoid molecule atisane
For molecules with a complicated 3-dimensional structure, especially involving atoms bonded to 4 dissimilar substituents, a simple molecular formula or fifty-fifty semi-structural chemical formula may not be enough to completely specify the molecule. In this case, a graphical type of formula chosen a structural formula may exist needed. Structural formulas may in turn exist represented with a one-dimensional chemical proper name, but such chemical classification requires many words and terms which are non office of chemical formulas.
Molecular geometry
Molecules have fixed equilibrium geometries—bond lengths and angles— about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the aforementioned average geometrical structure. The chemical formula and the structure of a molecule are the two of import factors that determine its properties, particularly its reactivity. Isomers share a chemical formula just normally have very unlike backdrop because of their different structures. Stereoisomers, a particular type of isomer, may have very like physico-chemical backdrop and at the same fourth dimension unlike biochemical activities.
Molecular spectroscopy
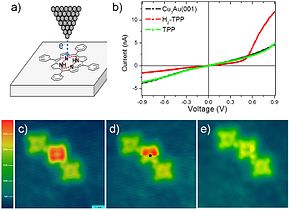
Hydrogen can be removed from individual H2TPP molecules by applying excess voltage to the tip of a scanning tunneling microscope (STM, a); this removal alters the current-voltage (I-V) curves of TPP molecules, measured using the aforementioned STM tip, from diode similar (reddish curve in b) to resistor similar (green curve). Image (c) shows a row of TPP, H2TPP and TPP molecules. While scanning image (d), excess voltage was applied to H2TPP at the black dot, which instantly removed hydrogen, every bit shown in the lesser part of (d) and in the rescan epitome (eastward). Such manipulations can be used in single-molecule electronics.[30]
Molecular spectroscopy deals with the response (spectrum) of molecules interacting with probing signals of known energy (or frequency, co-ordinate to Planck's formula). Molecules accept quantized energy levels that can be analyzed past detecting the molecule's free energy exchange through absorbance or emission.[31] Spectroscopy does not generally refer to diffraction studies where particles such as neutrons, electrons, or high energy Ten-rays collaborate with a regular arrangement of molecules (as in a crystal).
Microwave spectroscopy commonly measures changes in the rotation of molecules, and can exist used to identify molecules in outer infinite. Infrared spectroscopy measures the vibration of molecules, including stretching, angle or twisting motions. It is commonly used to identify the kinds of bonds or functional groups in molecules. Changes in the arrangements of electrons yield absorption or emission lines in ultraviolet, visible or near infrared light, and result in color. Nuclear resonance spectroscopy measures the environment of detail nuclei in the molecule, and can be used to characterise the numbers of atoms in different positions in a molecule.
Theoretical aspects
The report of molecules by molecular physics and theoretical chemistry is largely based on quantum mechanics and is essential for the understanding of the chemical bail. The simplest of molecules is the hydrogen molecule-ion, Hii +, and the simplest of all the chemical bonds is the ane-electron bond. H2 + is composed of two positively charged protons and one negatively charged electron, which ways that the Schrödinger equation for the organization tin can exist solved more easily due to the lack of electron–electron repulsion. With the development of fast digital computers, approximate solutions for more complicated molecules became possible and are one of the main aspects of computational chemistry.
When trying to define rigorously whether an arrangement of atoms is sufficiently stable to be considered a molecule, IUPAC suggests that information technology "must correspond to a depression on the potential energy surface that is deep enough to confine at least one vibrational state".[4] This definition does not depend on the nature of the interaction between the atoms, but just on the strength of the interaction. In fact, it includes weakly bound species that would not traditionally exist considered molecules, such every bit the helium dimer, Hetwo, which has one vibrational bound state[32] and is so loosely bound that it is only probable to be observed at very low temperatures.
Whether or not an system of atoms is sufficiently stable to be considered a molecule is inherently an operational definition. Philosophically, therefore, a molecule is not a fundamental entity (in contrast, for instance, to an elementary particle); rather, the concept of a molecule is the chemist's way of making a useful statement nearly the strengths of atomic-scale interactions in the earth that we discover.
See also
- Atom
- Chemic polarity
- Chemical construction
- Covalent bail
- Diatomic molecule
- List of compounds
- List of interstellar and circumstellar molecules
- Molecular biology
- Molecular blueprint software
- Molecular engineering
- Molecular geometry
- Molecular Hamiltonian
- Molecular ion
- Molecular modelling
- Molecular promiscuity
- Molecular orbital
- Non-covalent bonding
- Periodic systems of small molecules
- Small-scale molecule
- Comparison of software for molecular mechanics modeling
- Van der Waals molecule
- World Wide Molecular Matrix
References
- ^ Iwata, Kota; Yamazaki, Shiro; Mutombo, Pingo; Hapala, Prokop; Ondráček, Martin; Jelínek, Pavel; Sugimoto, Yoshiaki (2015). "Chemical structure imaging of a unmarried molecule by diminutive force microscopy at room temperature". Nature Communications. 6: 7766. Bibcode:2015NatCo...6.7766I. doi:x.1038/ncomms8766. PMC4518281. PMID 26178193.
- ^ Dinca, L.E.; De Marchi, F.; MacLeod, J.Thousand.; Lipton-Duffin, J.; Gatti, R.; Ma, D.; Perepichka, D.F.; Rosei, F. (2015). "Pentacene on Ni(111): Room-temperature molecular packing and temperature-activated conversion to graphene". Nanoscale. seven (7): 3263–9. Bibcode:2015Nanos...vii.3263D. doi:10.1039/C4NR07057G. PMID 25619890.
- ^ Hapala, Prokop; Švec, Martin; Stetsovych, Oleksandr; Van Der Heijden, Nadine J.; Ondráček, Martin; Van Der Lit, Joost; Mutombo, Pingo; Swart, Ingmar; Jelínek, Pavel (2016). "Mapping the electrostatic strength field of single molecules from high-resolution scanning probe images". Nature Communications. 7: 11560. Bibcode:2016NatCo...711560H. doi:10.1038/ncomms11560. PMC4894979. PMID 27230940.
- ^ a b IUPAC, Compendium of Chemic Terminology, 2d ed. (the "Aureate Book") (1997). Online corrected version: (2006–) "Molecule". doi:ten.1351/goldbook.M04002
- ^ Ebbin, Darrell D. (1990). General Chemistry (3rd ed.). Boston: Houghton Mifflin Co. ISBN978-0-395-43302-seven.
- ^ Brown, T.L.; Kenneth C. Kemp; Theodore L. Dark-brown; Harold Eugene LeMay; Bruce Edward Bursten (2003). Chemistry – the Central Scientific discipline (9th ed.). New Jersey: Prentice Hall. ISBN978-0-13-066997-one.
- ^ Chang, Raymond (1998). Chemistry (6th ed.). New York: McGraw Hill. ISBN978-0-07-115221-ane.
- ^ Zumdahl, Steven Due south. (1997). Chemistry (4th ed.). Boston: Houghton Mifflin. ISBN978-0-669-41794-4.
- ^ Chandra, Sulekh (2005). Comprehensive Inorganic Chemistry. New Age Publishers. ISBN978-81-224-1512-iv.
- ^ "Molecule". Encyclopædia Britannica. 22 January 2016. Archived from the original on 3 May 2020. Retrieved 23 February 2016.
- ^ Harper, Douglas. "molecule". Online Etymology Lexicon . Retrieved 22 February 2016.
- ^ "molecule". Merriam-Webster. Archived from the original on 24 Feb 2021. Retrieved 22 February 2016.
- ^ Molecule Definition Archived thirteen Oct 2014 at the Wayback Auto (Frostburg State University)
- ^ Ley, Willy (June 1966). "The Re-Designed Solar System". For Your Data. Galaxy Science Fiction. pp. 94–106.
- ^ Avogadro, Amedeo (1811). "Masses of the Unproblematic Molecules of Bodies". Journal de Physique. 73: 58–76. Archived from the original on 12 May 2019. Retrieved 25 Baronial 2022.
- ^ Seymour H. Mauskopf (1969). "The Atomic Structural Theories of Ampère and Gaudin: Molecular Speculation and Avogadro'south Hypothesis". Isis. 60 (one): 61–74. doi:x.1086/350449. JSTOR 229022. S2CID 143759556.
- ^ Perrin, Jean, B. (1926). Discontinuous Construction of Matter Archived 29 May 2019 at the Wayback Machine, Nobel Lecture, Dec eleven.
- ^ Heitler, Walter; London, Fritz (1927). "Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik". Zeitschrift für Physik. 44 (6–7): 455–472. Bibcode:1927ZPhy...44..455H. doi:10.1007/BF01397394. S2CID 119739102.
- ^ Pauling, Linus (1931). "The nature of the chemical bail. Awarding of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules". J. Am. Chem. Soc. 53 (four): 1367–1400. doi:10.1021/ja01355a027.
- ^ Harry, B. Gray. Chemical Bonds: An Introduction to Diminutive and Molecular Structure (PDF). pp. 210–211. Archived (PDF) from the original on 31 March 2021. Retrieved 22 November 2021.
- ^ "How many gold atoms make gold metal?". phys.org. Archived from the original on thirty Oct 2020. Retrieved 22 November 2021.
- ^ Campbell, Neil A.; Brad Williamson; Robin J. Heyden (2006). Biology: Exploring Life. Boston: Pearson Prentice Hall. ISBN978-0-13-250882-7. Archived from the original on two November 2014. Retrieved 5 Feb 2012.
- ^ Campbell, Scrap C. (2008). Elements of Metallurgy and Engineering Alloys. ASM International. ISBN978-1-61503-058-3. Archived from the original on 31 March 2021. Retrieved 27 Oct 2020.
- ^ Roger L. DeKock; Harry B. Greyness; Harry B. Gray (1989). Chemical structure and bonding. University Science Books. p. 199. ISBN978-0-935702-61-3. Archived from the original on 31 March 2021. Retrieved 27 Oct 2020.
- ^ Chang RL; Deen WM; Robertson CR; Brenner BM (1975). "Permselectivity of the glomerular capillary wall: Three. Restricted transport of polyanions". Kidney Int. 8 (4): 212–218. doi:10.1038/ki.1975.104. PMID 1202253.
- ^ Chang RL; Ueki IF; Troy JL; Deen WM; Robertson CR; Brenner BM (1975). "Permselectivity of the glomerular capillary wall to macromolecules. Ii. Experimental studies in rats using neutral dextran". Biophys. J. 15 (9): 887–906. Bibcode:1975BpJ....fifteen..887C. doi:x.1016/S0006-3495(75)85863-2. PMC1334749. PMID 1182263.
- ^ Flash, Donald J.; Fetzer-Gislason, Sharon; McNicholas, Sheila (2003). The Practice of Chemical science. Macmillan. ISBN978-0-7167-4871-7. Archived from the original on 10 Apr 2022. Retrieved 27 Oct 2020.
- ^ "ChemTeam: Empirical Formula". world wide web.chemteam.info. Archived from the original on 19 January 2021. Retrieved xvi April 2017.
- ^ Hirsch, Brandon Eastward.; Lee, Semin; Qiao, Bo; Chen, Chun-Hsing; McDonald, Kevin P.; Tait, Steven L.; Flood, Amar H. (2014). "Anion-induced dimerization of 5-fold symmetric cyanostars in 3D crystalline solids and 2D self-assembled crystals". Chemic Communications. 50 (69): 9827–30. doi:ten.1039/C4CC03725A. PMID 25080328. Archived from the original on 31 March 2021. Retrieved xx Apr 2018.
- ^ Zoldan, V. C.; Faccio, R; Pasa, A.A. (2015). "Northward and p type graphic symbol of single molecule diodes". Scientific Reports. 5: 8350. Bibcode:2015NatSR...5E8350Z. doi:10.1038/srep08350. PMC4322354. PMID 25666850.
- ^ IUPAC, Compendium of Chemical Terminology, 2d ed. (the "Gilded Book") (1997). Online corrected version: (2006–) "Spectroscopy". doi:10.1351/goldbook.S05848
- ^ Anderson JB (May 2004). "Comment on "An exact breakthrough Monte Carlo calculation of the helium-helium intermolecular potential" [J. Chem. Phys. 115, 4546 (2001)]". J Chem Phys. 120 (20): 9886–7. Bibcode:2004JChPh.120.9886A. doi:10.1063/1.1704638. PMID 15268005.
External links
- Molecule of the Month – School of Chemical science, University of Bristol
The Size Of A Molecule,
Source: https://en.wikipedia.org/wiki/Molecule#:~:text=Molecular%20size,-Most%20molecules%20are&text=Molecules%20commonly%20used%20as%20building,one%20billionth%20of%20a%20meter.
Posted by: bennettwasat1989.blogspot.com


0 Response to "The Size Of A Molecule"
Post a Comment