Are Exothermic Reactions Always Spontaneous
According to the Offset Police force of Thermodynamics, the full energy of an isolated system always remains constant. The first constabulary explains the relationship betwixt the piece of work done by the system or by the system and the estrus absorbed without putting any limitation on the direction of oestrus flow.
Tabular array of Contents
- What is Spontaneity?
- Recommended Videos
- Predicting the spontaneity of a reaction
- Gibbs Equation
- Frequently Asked Questions – FAQs
However, all processes which occur naturally tend to go along spontaneously in i direction simply. What does spontaneity mean here? What factors determine the direction of a spontaneous change?
What is Spontaneity?
Naturally, all processes take a tendency to occur in one direction under a given fix of conditions. A spontaneous process is an irreversible process and information technology could only exist reversed by some external agents. The entropy of any system is divers as the degree of randomness in it.
Recommended Videos

Predicting the spontaneity of a reaction
Generally, the total entropy change is the essential parameter which defines the spontaneity of any process. Since virtually of the chemic reactions autumn under the category of a airtight system and open system; we can say there is a change in enthalpy too along with the modify in entropy. Since, alter in enthalpy also increases or decreases the randomness past affecting the molecular motions, entropy change alone cannot business relationship for the spontaneity of such a procedure. Therefore, for explaining the spontaneity of a process nosotros use the Gibbs energy change. Gibbs' free energy is a land function and an extensive property. The full general expression for Gibbs energy alter at constant temperature is expressed every bit:
Gibbs Equation ⇒
ΔGsys = Δ Hsys – TΔ Ssys
Where,
ΔGsys = Gibbs energy modify of the organisation
ΔHsys = enthalpy change of the system
ΔSsys = entropy change of the organization
T= Temperature of the organisation
This is known every bit the Gibbs equation.
For a spontaneous procedure, the total entropy change, ΔSouthward total is always greater than zero.
ΔStotal=Δ Due southsys + Δ Southwardsurr
Where,
ΔStotal= total entropy modify for the process
ΔSsys = entropy change of the organization
ΔSsurr = entropy change of the surrounding
The modify in temperature betwixt the arrangement and the surround in the case of thermal equilibrium between the system and surround is 0, i.due east. ΔT= 0. Thus, enthalpy lost by the system is gained by the surrounding. Hence, the entropy change of the surrounding is given equally,
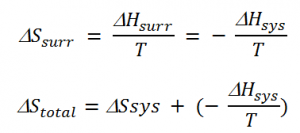
ΔHsurr = change in enthalpy of the surrounding
ΔHsys = alter in enthalpy of the organization
Also, for a spontaneous process, the total change in entropy is 0, i.e. ΔStotal> 0.
Therefore;
TΔSsys – ΔHsys>0
ΔHsys– TΔSsys<0
Using the Gibbs equation, it tin can exist said that
ΔGsys< 0
Thus, it can exist inferred that any process is spontaneous if the alter in Gibbs energy of the system is less than zero or else the procedure is not spontaneous.
Therefore, with the aid of the above relation, the spontaneity of a reaction can be hands predicted.
- In the case of exothermic reactions, the enthalpy of the system is negative thereby making all exothermic reactions spontaneous.
- In the case of endothermic reactions, Gibbs energy becomes negative but when the temperature is very high or the entropy change is very high.
Frequently Asked Questions – FAQs
How is a reaction spontaneous?
Reactions are favourable when they result in a decrease in enthalpy and an increment in entropy of the system. When both of these weather condition are met, the reaction occurs naturally. A spontaneous reaction is a reaction that favours the formation of products at the conditions under which the reaction is occurring.
Which chemical reaction is always spontaneous?
A reaction which is exothermic (ΔH negative) and results in an increase in the entropy of the system (ΔS positive) will ever be spontaneous.
Which procedure is spontaneous?
A spontaneous procedure is i that occurs on its own, without whatever energy input from the outside. For case, a brawl will roll downwards an incline; water volition flow downhill; ice will melt into water; radioisotopes will decay, and the iron volition rust.
Proper noun 2 factors which favour a spontaneous reaction.
Enthalpy is the total heat content of the system. Entropy is the measurement of randomness of the system. change in enthalpy and modify in entropy should be positive for a reaction to be spontaneous.
What is a spontaneous and non-spontaneous reaction?
A spontaneous process is capable of proceeding in a given direction without needing to be driven by an exterior source of energy. An endergonic reaction (besides chosen a nonspontaneous reaction) is a chemic reaction in which the standard change in complimentary energy is positive and free energy is captivated.
To acquire more about spontaneity and thermodynamics, register with BYJU'S and download our app – BYJU'South – The Learning App.
Are Exothermic Reactions Always Spontaneous,
Source: https://byjus.com/chemistry/spontaneity/
Posted by: bennettwasat1989.blogspot.com


0 Response to "Are Exothermic Reactions Always Spontaneous"
Post a Comment